Copied
DDW 2025: Key Takeaways on Nutrition Research in IBD
Dig into the latest research on nutrition, the gut microbiome, and IBD.

From May 3-6 2025, Nutritional Therapy for IBD attended Digestive Disease Week® in San Diego, USA, with more than 13,400 attendees (in-person and online). Dig into the latest research on nutrition, the gut microbiome, and IBD.
Urbanization and Westernized Diets Have Skyrocketed IBD Cases in Asia, Latin America, and the Middle East
Gilaad G. Kaplan, MD, MPH, and Joey Windsor, PhD, from the University of Calgary, shared findings from their 5-year journey defining the four-stage evolution of IBD1: emergence (stage 1), acceleration in new diagnoses made each year (incidence) (stage 2), increase in the number of people living with IBD (prevalence) (stage 3), and plateau of the number of people living with IBD where new diagnoses are balanced by age-related deaths (stage 4).

Source: Ashwin Ananthakrishnan’s presentation at DDW 2025.
While countries in North America, Europe, and Oceania are in Stage 3, developing areas in Africa, Latin America, and Asia are transitioning from Stage 1 to Stage 2 following economic growth, urbanization, and changes in lifestyle and diet. By 2045, developed countries will not only see more diagnoses of IBD but also a sharp increase in the number of people living with chronic disease, which implies managing parallel conditions such as cardiovascular diseases and dementia. North America, Europe, and Oceania are nowadays in Stage 3 and are facing new cases of IBD in young adults, while also supporting older patients with multiple and complex health needs.
While the causes of the disease are still unknown, Siew Ng, MBBS, PhD, from the Chinese University of Hong Kong, stressed that research should focus on preventing IBD by acting on environmental causes, such as diet. When populations move from a low to a high IBD area, they are exposed to the same disease risk as the host country, and their offspring have a similarly high presence of IBD compared to non-immigrants, involving a generational depletion of key microbiota strains associated with better health outcomes, but the reverse is also true.

Source: Ng’s presentation at DDW 2025.
Whole Food, High-Fiber Diets Offer Hope for Patients with Mild IBD
An only-liquid diet and oral nutritional supplements have shown efficacy for inducing remission in Crohn’s disease, but adherence challenges exist, and their impact on mental health disorders is unknown. Anissa Armet, PhD, RD, from the University of Alberta, showed three 5-day cycles of a plant-based, caloric-sufficient diet may improve symptoms of depression and fatigue more so than a fasting mimicking diet in patients with Crohn’s disease (CD) or ulcerative colitis (UC) who are already with IBD/depression medications. The fasting mimicking diet, consisting of a plant-based calorie-restricted diet consumed for five consecutive days a month while the diet returns to baseline for all other days, was more effective in improving digestive symptoms such as bloating, flatulence, and stomachaches, probably due to the reduced impact of food on digestion. The plant-based nature of diets, rather than whether or not they restrict calories, was the main factor probably driving changes at the level of gut microbiome functions, which might explain observed benefits on gut and mental health.

Source: Armet’s presentation at DDW 2025.
A randomized controlled trial presented by Chiraag V. Kulkarni, MD, from Stanford University, showed that a fasting mimicking diet induced clinical response and remission (a decline in Crohn’s Disease Activity Index for at least 70 points or CDAI less than or equal to 150) in patients with mild-to-moderate CD. There was a higher proportion of patients with at least a 50% decline in fecal calprotectin in the fast-mimicking group compared to the control diet, and four participants in the fast-mimicking diet were in endoscopic remission. Disease location also affected diet outcomes, with patients with ileocolonic and colonic CD showing a 60-80% clinical response, but not in isolated ileal disease. No difference in study withdrawals was found due to adverse events.

Source: Kulkarni’s presentation at DDW 2025.
Other diets high in fiber also offer hope for managing CD. Hajar Hazime, PhD, trained at at the Maria Abreu Lab at the University of Miami Health System Sylvester Comprehensive Cancer Center, revealed that patients with CD who ate a catered low-fat (20% of total calories), high-fiber diet (25-35 g fiber per day) (Mi-IBD diet) improved symptoms, quality of life, some inflammatory markers, and gut microbiome function as compared to those offered one time diet counselling. Responder patients and their caregivers also benefited from psychosocial support. The diet, which was previously tested in patients with UC, was well-tolerated after 8 weeks and led to increased enjoyment of food.2
Why Diet Quality is the Best Recipe for a Healthier Gut Microbiome in IBD
While healthy diets with adequate dietary fiber (>25g/day) are feasible in patients with mild IBD, downsides of fiber consumption exist. Heather Armstrong, PhD, from the University of Alberta, presented that fiber can impact host physiology differently, driven by the type and quantity of dietary factors. However, fiber can worsen health outcomes without the right microbes in the gut. It is the case of B-fructan and B-glucans, which can be pro- or anti-inflammatory depending on host immune status and gut microbiome function. It is also interesting to note that the benefits of dietary fiber are not always a result of its fermentation by gut microbes, as improving intestinal barrier function and immune reactions in the gut may also explain why not all fibers work equally. Matam Vijay-Kumar, PhD, from the University of Toledo, shared alarming data in mice showing that purified fibers, such as inulin and cellulose available in processed foods, are linked to intestinal atrophy and high liver cancer risk in mice, highlighting that food sources should be the go-to source of fiber for gut health.
Up to 40% of people with IBD are living with overweight or obesity, which is linked to worse symptoms, loss of treatment response, and increased post-operative complications. Natasha Haskey, PhD, RD, from the University of British Columbia-Okanagan, showed intermittent fasting may benefit some patients with CD in clinical remission or with mild disease activity, including an improvement in symptom burden (in particular stool frequency), a reduction of leptin, adipsin, and plasminogen activator-1 (molecules secreted by fat tissue that play roles in metabolism, inflammation, and heart health), and a better health-related quality of life. No changes in fecal calprotectin were observed. At baseline and week 12, patients with poor diets exhibited a gut microbiome enrichment with pro-inflammatory species, many of which were oral pathobionts. The takeaway is that time-restricted feeding is insufficient, and diet quality remains essential to support a healthy, anti-inflammatory gut microbiome.

Source: Haskey’s presentation at DDW 2025.
When it comes to dietary management of CD, Aaron Bancil, MBBS, PhD, from King’s College London, presented the first human trial showing that a low emulsifier is a safe and effective therapy for mild-to-moderately active CD, which was presented for the first time at the ECCO’25 Congress. Double-blind dietary trials using a re-supplementation methodology are not as ‘easy’ as pill vs. placebo trials. These findings highlight that diet interventions that intercept the disease course early to reduce symptoms and inflammation are as crucial as disease prevention tools.
Dietary Options for Patients with IBD-IBS Overlap
Up to one-third of patients with IBD in remission have IBS-type symptoms, which might be seen as a consequence of a Western diet high in ultra-processed foods and/or an altered microbiome, and are associated with higher health care utilization. An entire session at Digestive Disease Week focused on dietary treatment options for managing IBS symptoms, which affect many patients with quiescent IBD. Healthy eating advice based on the National Institute for Health Care Excellence (NICE) and British Dietetic Association (BDA) guidelines mainly focuses on how to eat and provides particular advice based on specific symptoms (e.g., increase intake of oats and flaxseed for bloating and flatulence) and should be the first choice.
Nancee Jaffe, MS, RDN, from the UCLA Vatche and Tamar Manoukian Division of Digestive Diseases, stated that the low FODMAP diet is the second-line treatment for IBS-like symptoms and is effective for pain and bloating. If diarrhea or constipation are the only predominant symptoms, the practitioner should investigate the cause. In patients with socioeconomic inability to follow a diet, those who are not interested in diet changes, those with a history of or active eating disorder, restricting only the most problematic FODMAPs (i.e., fructans, galactans, and mannitol) seems a reasonable approach.3,4

While gluten is one of the most critical culprits identified by patients, human data presented by Jessica Biesiekierski, PhD, RNutr, from the University of Melbourne, highlighted that available human studies show that FODMAPs, in particular fructans widely available in wheat, induce greater symptoms than gluten in people with IBS. However, gut symptoms are highly sensitive to stress, and this supports the need for integrated management of IBS that involves a gastroenterologist, a specialist dietitian, and a psychologist to help patients identify and manage food-induced gut symptoms in IBS5.
It is essential to highlight that even the right diet therapy does not work for patients with IBD who cannot even afford it. Social factors, such as access to food and housing, known as social determinants of health, affect individuals with IBD and could worsen their health issues. Individuals with IBD in the United States face a higher risk of food insecurity, with estimates of 13% vs. 8% in people without IBD, Berkeley N. Limketkai, MD, PhD, Mark R. Baniqued, MD, and colleagues from the UCLA School of Medicine in Los Angeles reported.
Microbiome-Based Therapeutics, Food Intolerances, and IBD
Modifying gut microbiome might impact IBD severity according to recent mice and human studies of fecal microbiota transfers (FMT) presented by Harry Sokol, PhD, MD, gastroenterologist at Saint Antoine Hospital and Sorbonne Université in Paris. While FMT boosts the good bugs in the gut, it is a treatment difficult to standardize, and many known and unknown components might drive its effects. To overcome this challenge, specific blends of bacteria strains from the human gut (bacteria consortia) can balance the altered microbiome and are moving into the clinic. On the other hand, a single bacterium might also be effective in fighting intestinal inflammation – this is the case of . When studying bug-based treatments for IBD, Sokol stressed that one should distinguish therapy for induction vs. maintenance and remember that a powerful immunosuppressant effect cannot be expected with microbiome-based drugs.6
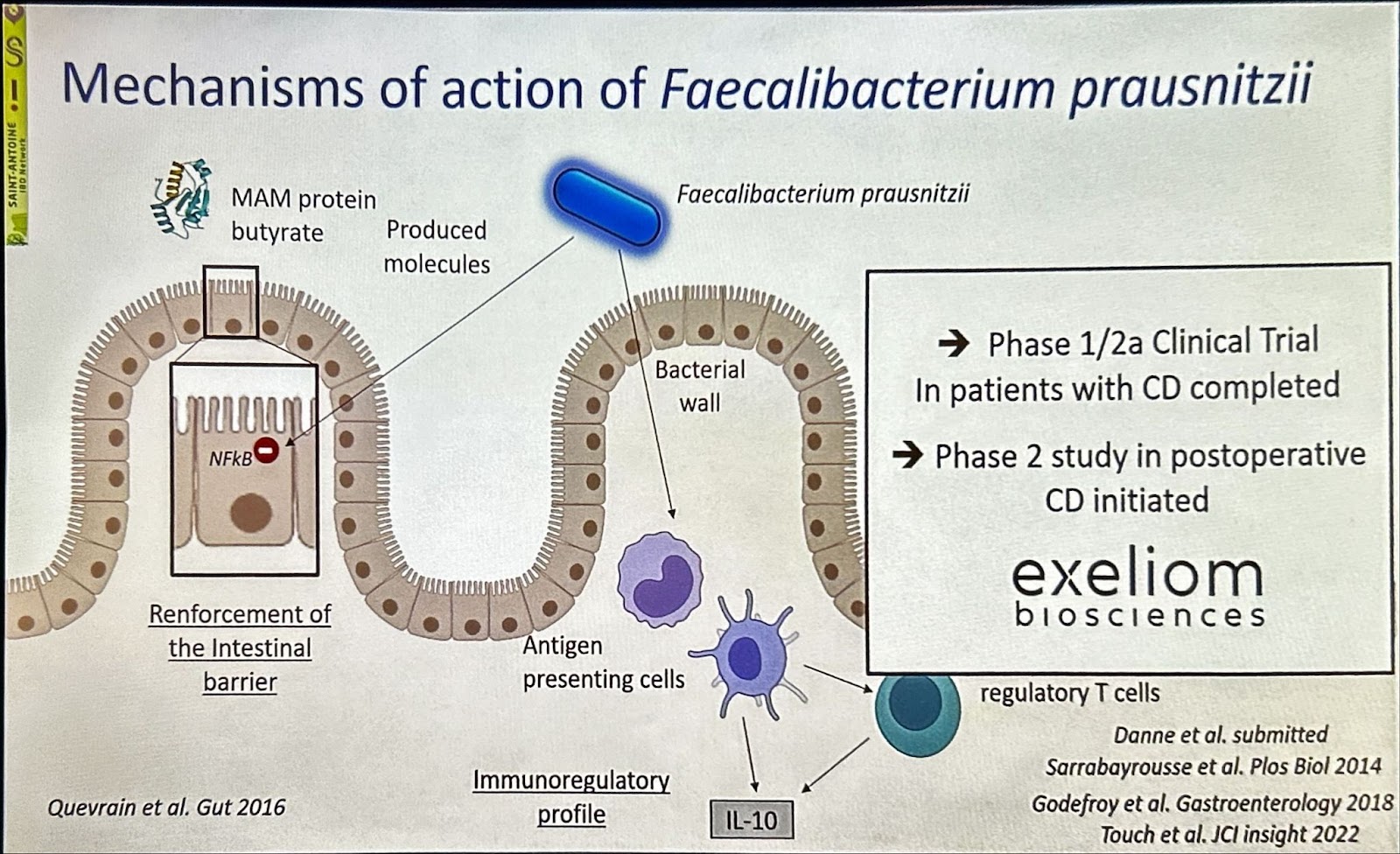
Source: Sokol’s presentation at DDW 2025.
The small intestine has the highest density of immune cells (especially mast cells), dietary antigen load, and greater availability of substrates for microbial metabolism. While the small intestine microbiome has been largely ignored due to its poor accessibility and challenges in studying it, new findings from Premysl Bercik, PhD, MD, from McMaster University, suggest its role in shaping intestinal inflammation and IBS-D symptoms. It turned out that neuroimmune abnormalities are more common in the small intestine, and bacterial phospholipids generated by inflammatory processes increase in IBS patients during periods of high pain and are potential targets for decreasing abdominal pain.7,8
Alberto Caminero, PhD, from Farncombe Family Digestive Health Research Institute, presented new data on the defective metabolic capacity of the microbiome as a relevant root involved in food sensitivities in IBD. Intestinal inflammation leads to alterations in microbial metabolism of offending foods in IBD. As a result, the immune system becomes sensitive to those allergens, which later can lead to allergy symptoms. The Caminero lab studies how gut inflammation facilitates sensitization to dairy proteins in mice by depleting bacteria that degrade dairy β-lactoglobulin and β-casein. To what extent gut microorganisms may boost immune reactions against other common offending foods in IBD (wheat, peanuts/tree nuts, caffeine, fiber, and FODMAPs) remains to be seen and opens the door towards using diet interventions that act on the microbiome for patients with IBD with adverse reactions to foods.9

Source: Caminero’s presentation at DDW 2025.
Digestive Disease Week® 2026 is scheduled for May 2-5 2026 in Chicago, USA.
Further Reading
1. Hracs L, Windsor JW, Gorospe J, et al. Global evolution of inflammatory bowel disease across epidemiologic states. Nature. 2025. doi: 10.1038/s41586-025-08940-0..
2. Mortera SL, Marzano V, Rapisarda F, et al. Metaproteomics reveals diet-induced changes in gut microbiome function according to Crohn’s disease location. Microbiome. 2024;12:217. doi: 10.1186/s40168-024-01927-5..
3.Eswaran S, Jencks KJ, Singh P, et al. All FODMAP’s aren’t created equal: results of a randomized reintroduction trial in patients with irritable bowel syndrome. Clin Gastroenterol Hepatol. 2025;23(2):351-358.e5. doi: 10.1016/j.cgh.2024.03.047.
4. Van den Houte K, Colomier E, Routhiaux K, et al. Efficacy and findings of a blinded randomized reintroduction phase for the low FODMAP diet in irritable bowel syndrome. Gastroenterology. 2024;167(2):333-342. doi: 10.1053/j.gastro.2024.02.008.
5.Chey WD, Keefer L, Whelan K, et al. Behavioral and diet therapies in integrated care for patients with irritable bowel syndrome. Gastroenterology. 2021;160(1):47-62. doi: 10.1053/j.gastro.2020.06.099.
6. Benech N, Sokol H. Targeting the gut microbiota in inflammatory bowel diseases: where are we? Curr Opin Microbiol. 2023;74:102319. doi: 10.1016/j.mib.2023.102319
7. Pujo J, De Palma G, Lu J, et al. Signaling mechanisms underlying nociceptive effects of bacterial lysophosphatidylcholine (LPC) and lysophosphatidic acid (LPA) [Guided Poster Session, Su1775] Digestive Disease Week® (DDW) 2025, San Diego.
8. Vanuytsel T, Bercik P, Boeckxstaens G. Understanding neuroimmune interactions in disorders of gut-brain interaction: from functional to immune-mediated disorders. Gut. 2023;72(4):787-798. doi: 10.1136/gutjnl-2020-320633.
9. Caminero A, Meisel M, Jabri B, et al. Mechanisms by which gut microorganisms influence food sensitivities. Nat Rev Gastroenterol Hepatol. 2019;16(1):7-18. doi: 10.1038/s41575-018-0064-z

Andreu Prados. BS. Pharm, RD, PhD is a science and medical writer specializing in making reliable evidence of non-prescription therapeutics for gastrointestinal conditions understandable, engaging and ready for use for healthcare professionals and patients.
Stay Informed of the Latest News in Nutrition and IBD.




Support our Mission
Your donation will help us to enhance the well-being and health outcomes of patients with GI conditions.
Donate Donate
Donate
